Recently, The team of Chen Jianhua & Chen Ye from SKL is entitled "An in-situ study of the interaction mechanism between xanthate and unactivated/Cu-activated sphalerite surfaces at solid–liquid interfaces" was published in the Applied Surface Science.
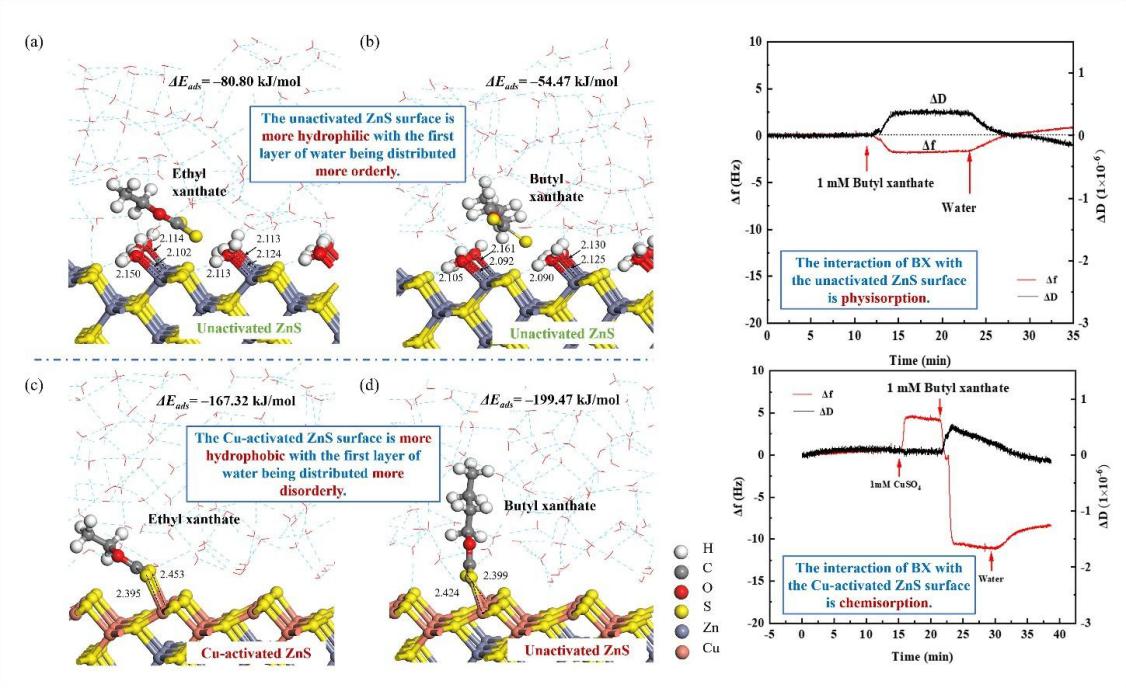
The mechanism of surface hydration and xanthate adsorption in the flotation of unactivated/Cu-activated sphalerite is still controversial. Therefore, a study combining in-situ measurement and quantum simulation is employed to investigate the interaction mechanism of ethyl xanthate (EX) and butyl xanthate (BX) on the fully hydrated, unactivated/Cu-activated sphalerite surfaces at solid–liquid interfaces. Density functional based on tight-binding (DFTB+) and molecular dynamics (MD) results suggest that the Cu-activated surface is hydrophobic with the first layer of water being distributed more disorderly on it. This is due to the difficulty of the lone pair electrons of water to interact with Cu. On the unactivated surface, water adsorption is more favorable than xanthates, hence, water molecules are physically adsorbed on it. Whereas the interaction of EX/BX with the hydrated, activated surface is chemisorption. The higher reactivity of Cu+ d orbitals to form π-backbonds with xanthate and the orbital symmetry matching of the Cu dyz orbitals and xanthate pz orbitals contribute to these results. Microcalorimetric results also demonstrate that copper activation enhances the exothermic heat and reaction rate, facilitating the interaction of EX/BX with sphalerite. These are consistent with the flotation and quartz crystal microbalance with dissipation (QCM-D) results.