Recently, The team of Professor Li Jing from SKL is entitled "A novel LDHs-VB3- inhibitor for rebar corrosion in simulated concrete pore solution: Synthesis, performance and mechanism" was published in the Composites Part B: Engineering.
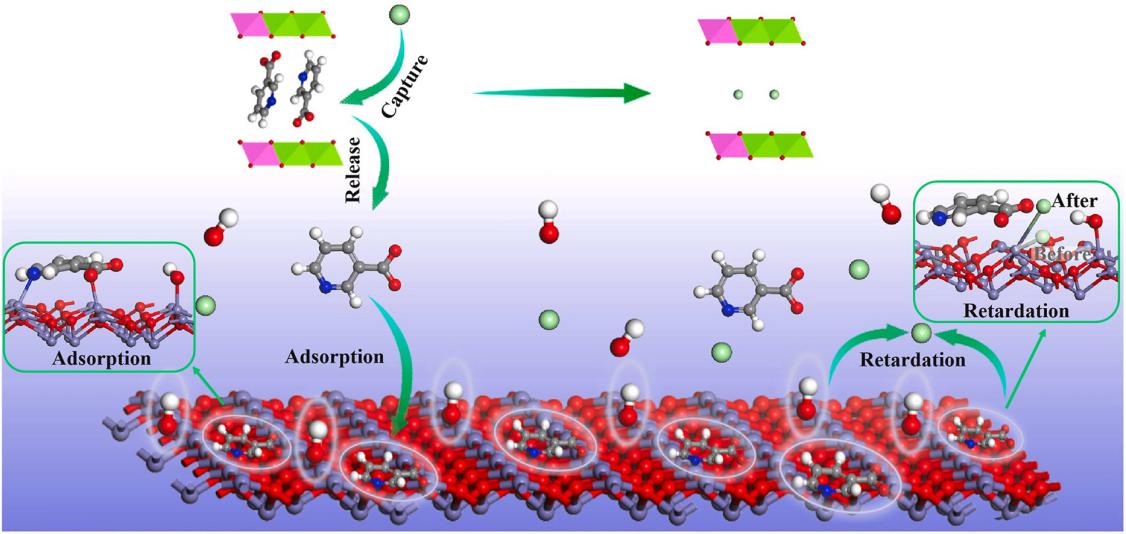
Developing novel chloride-trapping inhibitors is of great significance for enhancing the durability of marine concrete structures. In this study, a novel LDHs-VB3- inhibitor was synthesized through the ion exchange method. Then, the corrosion inhibition mechanism of LDHs-VB3- inhibitor for rebar in the whole process of release - capture - adsorption - retardation was studied using experiments and density functional theory (DFT) simulations. The chloride ion exchange test demonstrates that the LDHs-VB3- inhibitor has a chloride trapping capacity of 35.5 mg/g in the saturated Ca(OH)2 solution containing 0.14 M NaCl, implying that it could capture Cl- in concrete pore solution. According to the electrochemical tests, the critical chloride concentration of rebar in the simulated concrete solution containing LDHs-VB3- falls within a range of 0.22 M–0.28 M, which is much higher than that in the solution without LDHs-VB3- (0.10 M–0.14 M). DFT simulations indicate that LDHs-VB3- exhibits more positive binding energy and uneven binding forces in contrast to LDHs-Cl-. Consequently, VB3- could be easily replaced by Cl- and then released into the pore solution of concrete. Moreover, the released VB3- could adsorb on the α-Fe2O3 surface in a concrete environment via the interaction of surface Fe atoms with N and O atoms of VB3-. The preadsorbed VB3- could diminish the interaction of Cl- with the α-Fe2O3 surface by reducing electron transfer, thereby mitigating the risk of passive film breakdown and retarding the chloride-induced corrosion of rebars.