Recently, The team of Yin Shibin from SKL is entitled "Synergistic modulation of hydrogen bond network reconstruction and pH buffering of electrolyte enables highly reversible Zn anode" was published in the Chemical Engineering Journal.
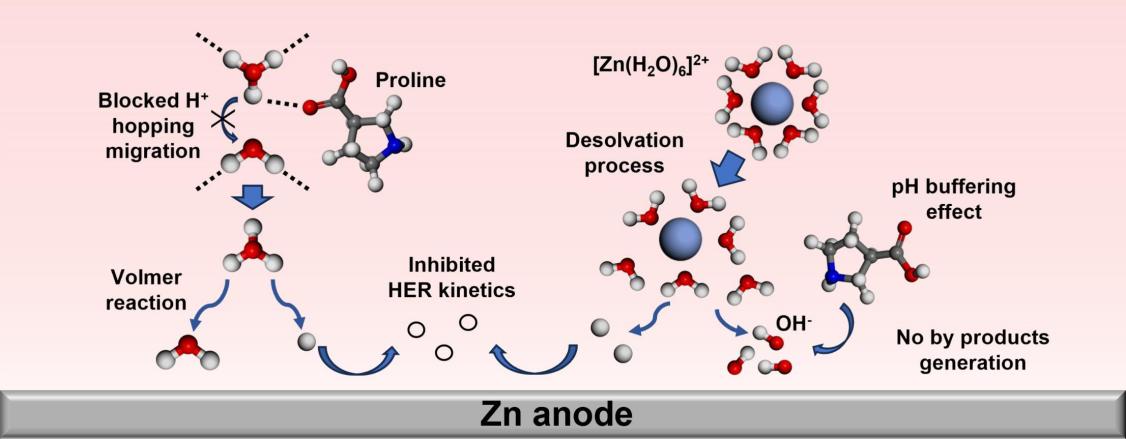
Utilizing aqueous electrolytes offers zinc-ion batteries (ZIBs) with inherent safety, high ionic conductivity, and power density, which is promising for energy storage applications. While the H2O-induced problems including hydrogen evolution reaction (HER) and the accumulation of by-products hinder the electrochemical performance of ZIBs. Herein, proline is used as an electrolyte additive to solve the above issues. Density functional theory (DFT) calculation and experimental results confirm that abundant hydrogen bonds (HBs) are formed between proline and H2O, disrupting the original HB network to inhibit HER kinetics. Meanwhile, as an amphoteric compound, proline neutralizes the OH− generated by HER, preventing it to react with Zn2+ and SO42− to form insulating by-products. Consequently, the Zn||Zn symmetric cell reaches the cycle life of 5500 h at 1.0 mA cm−2, 1.0 mAh cm−2, and 660 h at 10 mA cm−2, 5.0 mAh cm−2. When matching with the VO2 cathode, the Zn||VO2 cell achieves a capacity retention of 68.25 % at 5.0 A g−1 after 6694 cycles. This work provides a novel perspective integrating the regulation of the HB network and pH buffer chemistry to develop advanced aqueous electrolytes.