Recently, The team of Professor Yin ShiBin from SKL is entitled "Built-in electric field in NiO-CuO heterostructures to regulate the hydroxide adsorption sites for 5-hydroxymethylfurfural electrooxidation assisted hydrogen production" was published in the Journal of Colloid and Interface Science.
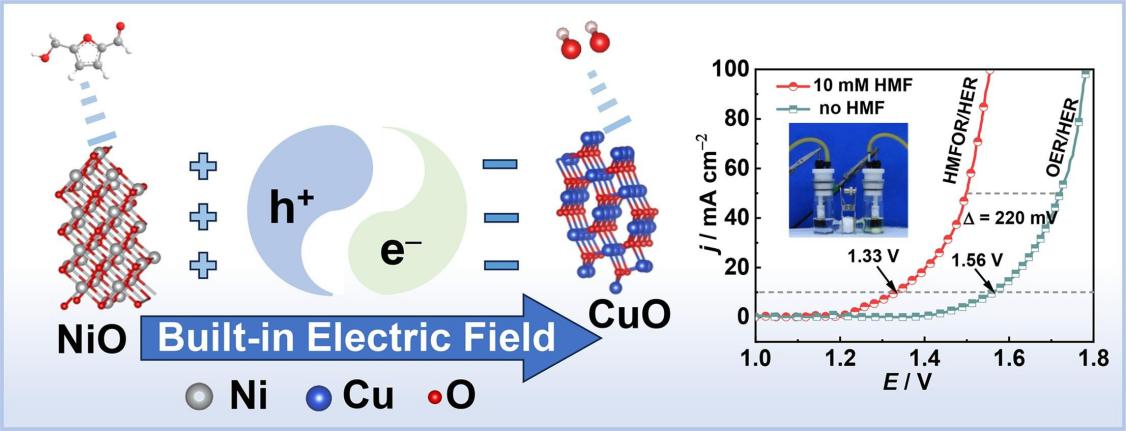
The development of catalysts with suitable adsorption behavior for the reaction molecules and the elucidation of their internal structure-adsorption-catalytic activity relationships are crucial for the electrooxidation of 5-hydroxymethylfurfural (HMF). In this work, NiO-CuO heterostructures with a spontaneous built-in electric field (BEF) are specifically designed and used to regulate the OH− adsorption site for freeing up the active site of HMF for the HMF oxidation reaction (HMFOR). The mechanism driving electron pumping/accumulation of the BEF is examined by X-ray photoelectron spectroscopy (XPS) and ultraviolet photoelectron spectroscopy (UPS). Electrochemical data and theoretical calculations show that BEF modulates the adsorption energy and adsorption site of substrate molecules, thereby enhancing the performance of HMFOR and hydrogen evolution reaction (HER). Notably, the NiO-CuO electrode demonstrates high 2,5-Furandicarboxylic acid (FDCA) selectivity (99.76 %) and generation rate (13.79 mmol gcat−1 h−1). It only requires 1.33 V to obtain a current density of 10 mA cm−2 for HMFOR-coupled H2 evolution. This research introduces a novel approach by regulating the adsorption of reactive molecules for HMFOR-assisted H2 evolution.